
Featured Speakers:
Denny Lorenz
Managing Director and Principal Consultant
Lorenz Bratti GmbH

Product Compliance
Streamline product lifecycle management and ensure data submission compliance.
Product Compliance
Streamline product lifecycle management and ensure data submission compliance.
LifeSphere® Technology is trusted by these industry leaders:




Product Compliance
Product Compliance enables efficient management and exchange of product data according to xEVMPD/IDMP requirements.
Standardized Processes
Standardize product data governance and lifecycle management.
Single Source of Truth
Enable increased consistency and quality of data via a controlled and centralized environment for medicinal product data facilitating reuse, impact assessment and data updates.
Enhanced Compliance
Ensure adherence to agency requirements and compliance with xEVMPD and IDMP requirements without the need for technical knowledge.
Process Automation
Automate the creation and update of medicinal products as part of the overall RIM lifecycle.
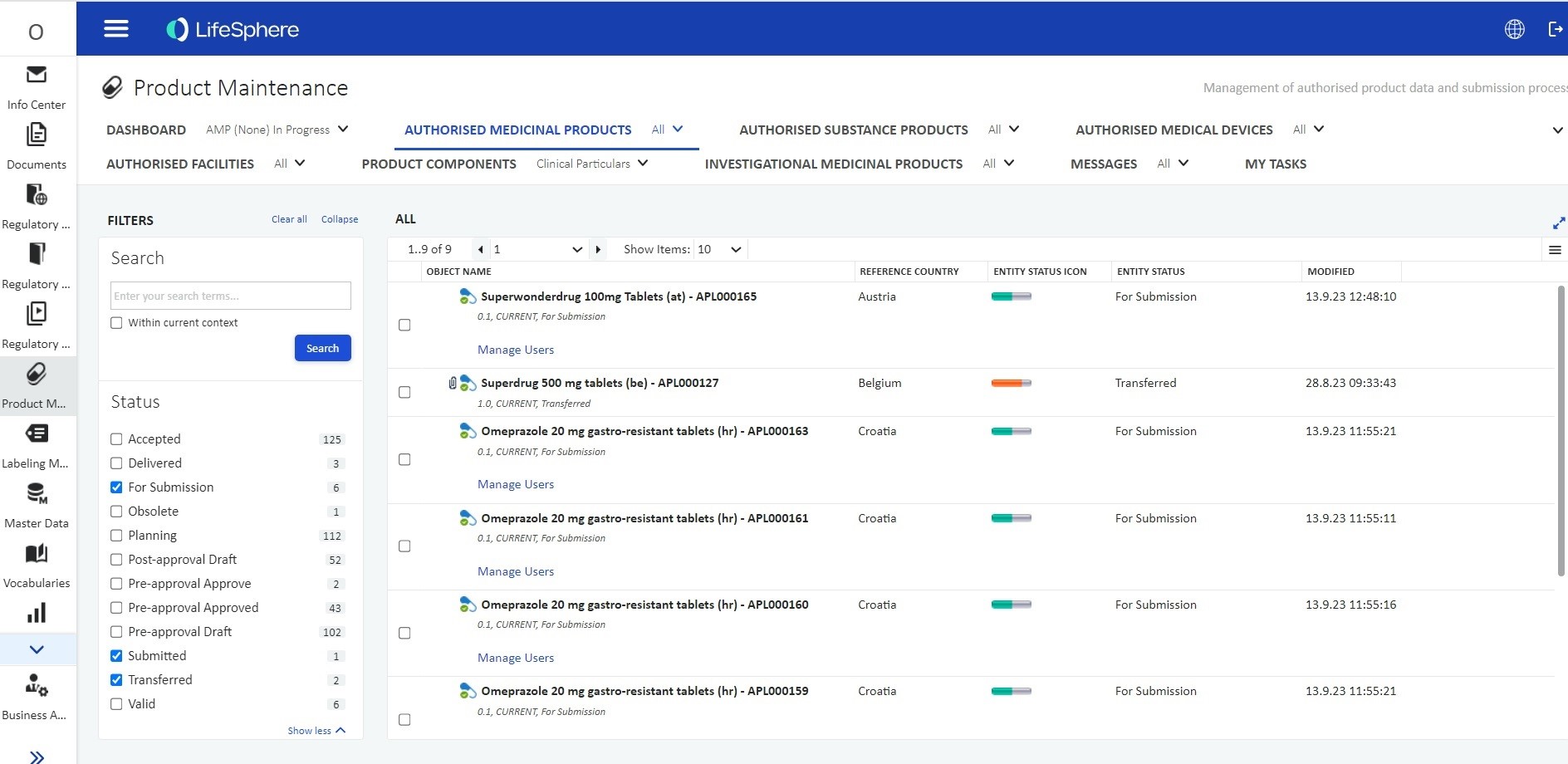
Features
Deliver real value with Products Compliance.

Product Data Management
Enhance product data standardization and lifecycle management.

xEVMPD Support
Embedded workflows for xEVMPD submission and data maintenance.

IDMP Compliance
Built-in IDMP support and validation according to the regional Implementation Guidelines.

Interoperability
Utilize direct gateway connectivity and SPOR integrations.

Data Enrichment
Automate data extraction and validation for enhanced data quality.

Interactive Dashboards
Gain actionable insights into managing product information.
Related Resources
Learn more about our collaborative partnership with customers.
"*" indicates required fields

ArisGlobal is a pivotal partner in the life sciences industry, specializing in solutions that drive drug development, safety monitoring, and regulatory compliance. We collaborate with more than 220 global life sciences companies, CROs, and government health authorities, including leading biopharmaceutical firms and regulatory bodies such as FDA, Health Canada, and NMPA.
Headquartered in the United States, ArisGlobal has regional offices in Europe, India, Japan and China.
Quick Links
© 2024 ArisGlobal – All Rights Reserved